Carbon storage, also known as carbon sequestration, is a complex method of capturing carbon dioxide emissions and storing them in coal seams, aquifers, depleted oil and gas reservoirs and other spaces deep under the surface of the Earth. In simple words, it’s the intake and storage of the element carbon.
The most common example in nature is during the photosynthesis process of trees and plants, which store carbon as they absorb carbon dioxide (CO2) during growth. Because they soak up the carbon that would otherwise rise up and trap heat in the atmosphere, trees and plants are important players in efforts to stave off global warming in a process called climate change mitigation.
In order for ocean storage of carbon dioxide to become a workable strategy for mitigating climate change, certain criteria must be met:
1) sequestration technologies must be cost-effective
2) environmental impacts must be acceptable
3) techniques must be politically and legally feasible
4) scientific certainty must be increased as to the effectiveness of the oceans to safely sequester CO2
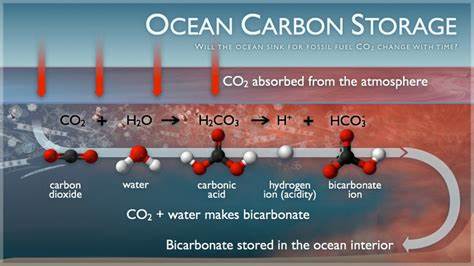
The way in which CO2 is stored in the ocean depends on its phase state and the depth at which it is injected:
| Ocean Depth | Phase State |
| 500 meters | Gas |
| 2700 meters | Liquid |
| 3000 meters | Solid |
Carbon dioxide is naturally stored in the ocean through chemical processes, either as a dissolved gas or, over a longer time scale, as carbonate sediments on the seafloor. Storage of CO2 in the ocean accelerates this natural storage process. It transports CO2 from the atmosphere to the deep ocean, where it will eventually wind-up anyways, and do less harm.
Efficacy and concerns:
The effectiveness of ocean storage of CO2 depends on how long the stored CO2 remains isolated from the atmosphere. Ocean currents carry surface waters to the deep and vice versa. This mixing effect is more pronounced near the surface and generally decreases with depth.
Another perceived advantage of ocean storage is capacity and availability. According to the International Energy Agency’s Greenhouse Gas R&D Programme the world’s hydrocarbon reservoirs have a combined storage capacity of roughly 800 gigatons of CO2 (GtCO2). In other words, terrestrial storage could handle about 22 years worth of emissions at our current levels. Ocean storage of CO2 could also buy us more time with our carbon budget, which is the amount of carbon that we can emit into the atmosphere without causing a two-degree Celsius temperature change.
While ocean storage of carbon dioxide mitigates climate change, there is the obvious issue of ocean acidification. Ocean Acidity is an indicator which describes changes in the chemistry of the ocean that relate to the amount of carbon dioxide dissolved in the water. The ocean plays an important role in regulating the amount of carbon dioxide in the atmosphere. As atmospheric concentrations of carbon dioxide rise, the ocean absorbs more carbon dioxide and that declining pH can have a negative effect on some marine life.
Environmental Impact of Carbon dioxide (CO2) sequestration:
The IPCC expects that annual greenhouse gas emissions will double in the next 50–100 years. This will result in a cascade of environmental effects such as:
- melting of polar ice and oceanic expansion
- increase in the number and severity of tropical storms and cyclones
- flooding and erosion of agricultural plot
- major shifts in ecosystems and decreasing
- biodiversity
Alternatives:
In order to avoid the above mentioned dramatic environmental effects, there are three strategies for lowering CO2 emissions and mitigating climate change (Schrag, 2007):
- reduction of global energy use
- development of low or no-carbon fuel
- sequestration of CO2 from point sources or atmosphere through natural and engineering techniques.
If we want to mitigate climate change, we need to reduce the amount of carbon dioxide in the atmosphere. One strategy to rapidly decarbonize and reduce CO2 emissions is through the adoption of renewable energy and improved energy efficiency, but this is not enough. In addition to decarbonization we need to remove excess CO2 from the atmosphere. While oceans are actually one of the largest sinks on the planet, in the process the atmosphere and ocean surface waters will be subjected to unprecedented warming and acidification.